 Relevancy and Engagement
agclassroom.org/ia/
Relevancy and Engagement
agclassroom.org/ia/
Lesson Plan
Blue's the Clue: Souring Milk for Science (Grades 6-8)
Grade Level
Purpose
This lab introduces students to the effect temperature has on reducing and controlling the growth of bacteria. Students will use conventionally pasteurized and ultra-high-temperature (UHT) milk to observe how different temperatures (hot, room temperature, cool, and freezing) affect the growth of spoilage bacteria. They will also learn about the importance of pasteurization in keeping food safe. Grades 6-8
Estimated Time
Materials Needed
For the Class:
- Refrigerator with freezer compartment, if possible
- Dr. X and the Quest for Food Safety Module III: Processing and Transportation video
For Each Team:
- 30 ml of pasteurized 2% milk (10 ml/test tube)
-
30 ml of shelf-stable ultra-high-temperature (UHT) 2% milk (10 ml/test tube)*
- Methylene blue dilute solution (1 drop per test tube)*
- 6 sterile test tubes with caps or aluminum foil to cover the test tubes*
- Small pipette or graduated cylinder for measuring out milk
- Permanent marker to label test tubes
- Blue’s the Clue Data Table, 1 per team or student
*These items can be purchased in the Blue’s the Clue Kit from agclassroomstore.com.
Advance Preparation
- Order lab supplies.
- Purchase pasteurized 2% milk and ultra-high-temperature (UHT) 2% milk. (UHT milk is shelf-stable and can often be found in the juice aisle or sometimes in small cartons with other milk; look for the UHT label. Ask your store manager to order it if it isn’t available in your supermarket or purchase UHT milk from agclassroomstore.com.)
- Make one copy of the attached Blue’s the Clue Data Table for each student.
- On activity day, place all the equipment on a lab table.
Vocabulary
ultra high temperature (UHT) milk: milk heated to at least 280° F (138° C) for 1 or 2 seconds, then packaged in sterile, airtight containers, making it shelf stable or able to be stored without refrigeration
Did You Know?
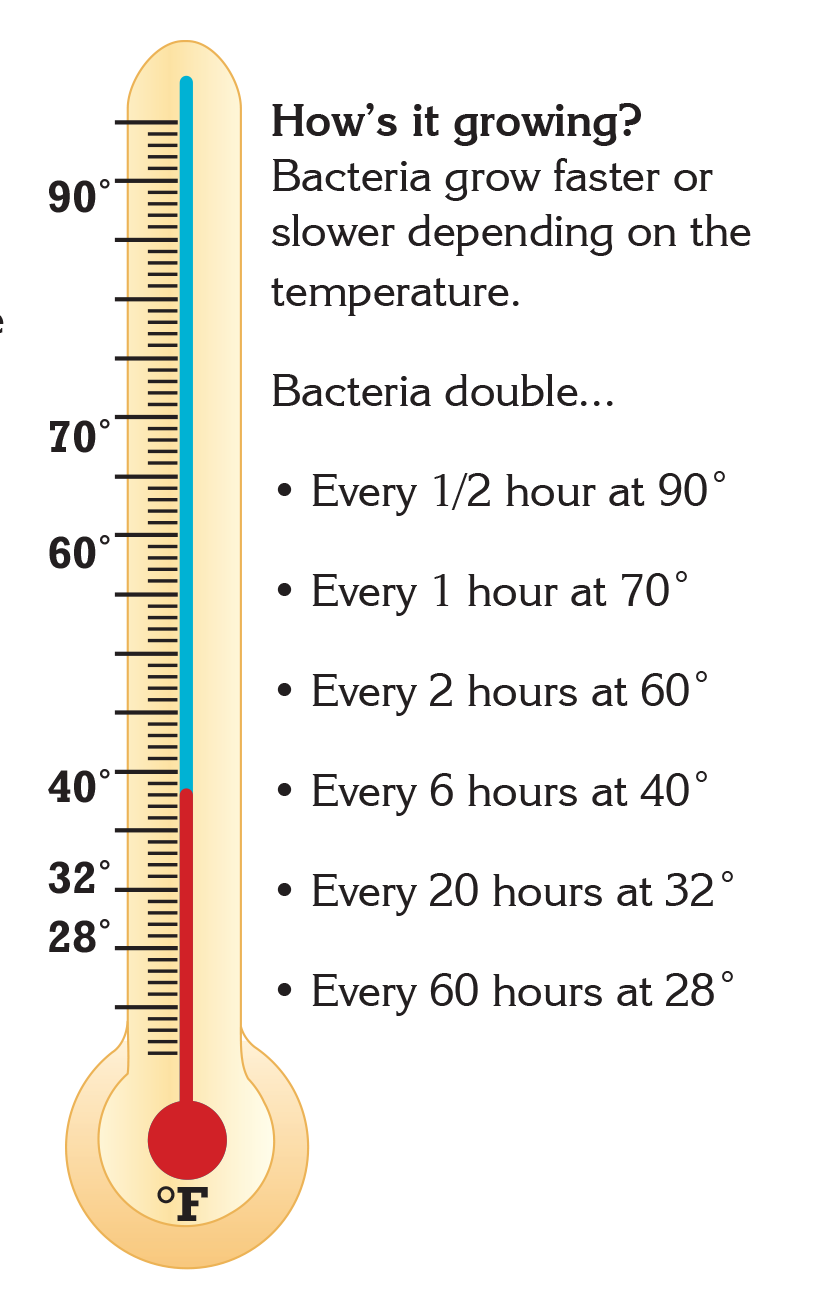
- Bacteria grow most rapidly in the range of temperatures between 40°F and 140°F.1
- This range of temperatures is often called the "Danger Zone" for food.1
- At Danger Zone temperatures, the bacteria that contaminate food and cause illness can double in number in just 20 minutes.1
Background Agricultural Connections
Did you know that there are many ways to limit and control the presence of bacteria in food? Keeping food at safe temperatures is one of the easiest ways to prevent the growth of bacteria that cause illness. Leaving food out at room temperature for too long can put the food into "Danger Zone" temperatures between 40°F and 140°F. In this temperature range, common bacteria that cause illness can double in number in just 20 minutes. At home, it is important to remember to keep cold food cold and hot food hot. However, even before food gets to your home, many foods are processed to reduce or eliminate the presence of potentially harmful bacteria.
Much of the food processing that happens between the farm and the grocery store aims to keep our food safe. This lesson focuses on conventionally pasteurized and ultra-high-temperature (UHT) milk. Pasteurization and UHT treatment are different ways of using heat to process milk in order to reduce the presence of bacteria. By learning about how temperature affects the growth of bacteria, students will be able to better understand how they should prepare and store food at home to help reduce bacterial growth.
Pasteurization
The technology of dairy science is continually improving, but some fundamentals remain the same. Bacteria are ubiquitous, and the bacteria present in milk can grow quickly in number, causing the milk to spoil or causing us to get sick. Pasteurization is a heat treatment performed at the milk processing plant which destroys harmful bacteria without affecting the quality of the milk. Milk may be pasteurized to kill bacteria using a low heat method (145°F, 63°C for 30 minutes) or a high heat method (162°F, 72°C for 15 seconds). Pasteurization does not kill all the bacteria contained in raw milk, but it does kill those pathogens that may cause disease. Bacteria that remain after pasteurization eventually cause milk to sour (spoil). Pasteurized milk is refrigerated to slow the growth of spoilage bacteria.
Ultra-high-temperature (UHT) treatment methods, as used by Gossner Foods in northern Utah, create milk that is stable at room temperature for nearly as long as the packaging remains sealed. UHT milk is heated to at least 280°F (138°C) for 1 or 2 seconds, then packaged in sterile, airtight containers. Because of the high heat and special packaging, UHT milk contains fewer bacteria than conventionally pasteurized milk. UHT milk is shelf stable and can be stored without refrigeration for up to 90 days. After opening, spoilage time for UHT milk is similar to that of conventionally pasteurized milk. Therefore, after opening, it should be refrigerated just like pasteurized milk.
Quality Control and Dairy Careers
Quality control workers in milk processing plants check for bacteria in the milk before they load it into a truck, again before the truck is unloaded at the processing plant, and in the storage tank at the processing plant before and after the milk is pasteurized. A quality control worker will continue to test the milk lots daily for 10 days after they are bottled. There are two minimum tests that must be conducted: 1) check the concentration of microorganisms in raw and pasteurized milk, and 2) check and detect the viable and dead microorganisms.
Careers in the dairy field encompass everything from quality control workers who ensure that the milk processing procedures are clean and safe for consumers, to breeders and geneticists working to increase milk yields of individual dairy cows, to scientists and researchers who are continually improving safety methods and maximizing productivity.
Souring Milk for Science
In UHT and pasteurized milk that are beginning to spoil, various microorganisms succeed one another as the chemical environment of the milk changes. The microbes themselves bring about these changes. The stages of microbial growth are dominated by different microorganisms. First, the population of Streptococcus bacteria grows rapidly, then Lactobacillus, then yeasts and molds, and finally Bacillus. Streptococci convert the milk sugar (lactose) into lactic acid. The acidity of the milk increases to the point where further Streptococci growth is inhibited. Lactobacilli then begin to grow and convert the remaining lactose into lactic acid. Acidity increases further until Lactobacilli growth is suppressed. The lactic acid sours the milk and curdles (coagulates) the milk protein. Yeasts and molds grow well in this acidic environment, and they convert acid into non-acid products. Finally, Bacilli multiply in the environment where protein is the only nutrient available.
Bacilli convert protein into ammonia products, and the pH (acidity) rises. These bacteria also digest the remaining protein through enzymatic action. The odor of spoiled milk becomes apparent once this has happened. Microbial activity causes changes in the pH of the milk. Fluctuations in pH are caused by fermentation and putrefaction (decomposition) processes.
As bacterial populations grow, they begin to use up the oxygen in the milk. Adding methylene blue to milk will turn it a blue color, and it will remain blue as long as oxygen is present in the milk. The more bacterial activity there is, the faster the oxygen will be used, and the sooner the milk will turn white, indicating that it is spoiled. For the following activities, you will be monitoring the speed at which the milk changes from blue to white and making comparisons between the different methods of pasteurization described above.
This rudimentary experiment will give you a sense of the differences between milk pasteurization methods, but it is not typically used by scientists to test the quality of milk. Rather, quality control studies are performed by examining the bacteria present in the milk, ensuring higher accuracy than the blue test and helping workers to find the source of bacterial contamination.
Why Pasteurize?
There are risks to be considered when drinking raw, unpasteurized milk. Raw or unpasteurized milk can carry dangerous bacteria such as Salmonella, E. coli, and Listeria, which are responsible for causing numerous foodborne illnesses. These harmful bacteria can seriously affect the health of anyone who drinks raw milk, or eats foods made from raw milk. However, the bacteria in raw milk can be especially dangerous to pregnant women, children, the elderly, and people with weakened immune systems. Pasteurization kills these pathogenic or disease-causing microorganisms.
About Methylene Blue
Methylene blue is an indicator dye used in this experiment. In anaerobic conditions it is reduced to leucomethylene and becomes colorless. When methylene blue is added to fresh milk, it will dye the milk blue. As bacterial populations grow in the milk, they will use up the oxygen, and the methylene blue will lose its color. The rate at which the milk loses its color is a relative measure of bacteria present in milk.
 Science and our Food Supply
Science and our Food Supply
This lesson was developed as a piece of a larger unit of lessons focusing on food safety from farm to table. Use the following links to see the other lessons in the unit:
Module 1: Bacteria
Module 2: Farm
Module 3: Processing and Transportation
- Blue's the Clue: Souring Milk for Science
- Mystery Juice
- Ultra High Pressure Treatment
Module 4: Retail and Home
- Supermarket Smarts
- Cooking Right: The Science of Cooking a Hamburger
- A Chilling Investigation
- Crossed Up!
- Hands Off Bacteria!
Module 5: Outbreak and Future Technology
Evaluation: Lose a Million Bacteria (The Game)
Engage

- Brainstorm with students all of the careers or jobs involved in the production of milk and dairy products (cheese, yogurt, and ice cream). List careers on the board. Begin on the farm and progress through the transportation, processing, and distribution of milk. Prompt students as they are brainstorming, and fill in the gaps as you go.
- Farm: dairy farmer, animal nutritionist, engineer, veterinarian, etc.
- Processing plant: processing plant workers, quality control managers, food scientists, etc.
- Transportation: truck drivers, diesel mechanics, etc.
- Retail: grocery store workers, managers, etc.
- With all of the dairy-related careers listed on the board, circle the quality control manager. (You will likely need to list this one yourself).
- Ask students if they have ever wondered why their parents are always asking them to put the milk back in the refrigerator. What might happen to that milk if it were left out at room temperature overnight? Reference the information at the beginning of the Background Agricultural Connections section of the lesson to teach students about the food "Danger Zone." Explain to students that quality control managers make sure that milk never enters the Danger Zone as it is being processed and transported from farm to store.
- Let students know that they will be learning about the science of microbes and about careers in the food science industry. It is important to monitor and control our food supply in order to avoid foodborne illness caused by microbes that might make us sick.
- Ask your students what pasteurization is. Use the information found in the Background Agricultural Connections section of the lesson to teach them about conventional pasteurization and ultra-high-temperature (UHT) treatment.
Explore and Explain
- Prepare students for a video clip by explaining that after food leaves the farm, it is typically processed in some way. This could include washing, packaging, cooking, and/or other means of preparing food for retail sale and eventually consumption.

- Inform students that "Dr. X" will beam them into the research lab of one of his scientist friends who looks at new ways to reduce the bacteria in our food through processing. Here are some things they should think about while they watch the video:
- What do cows, astronauts, and elephants have to do with food safety and food processing? (Cows relate to pasteurization, astronauts relate to irradiation, and elephants relate to ultra-high-pressure treatment.)
- What is pasteurization? (Pasteurization uses heat to kill harmful bacteria in foods.)
- How can an egg be pasteurized in the shell without cooking it? (Manufacturers use a time/temperature relationship to pasteurize eggs in the shell without cooking them. Heating eggs above 140° F [60° C] will cook them. Thus, using a lower temperature of 135° F [57° C] for a longer time [1 hour and 15 minutes] kills bacteria without cooking the egg.)
- What process keeps food safe in outer space? (Irradiation)
- Show video Dr. X and the Quest for Food Safety Module III: Processing and Transportation (Time: 7 minutes).
- Discuss the role of refrigeration in keeping foods safe. Ask students how some types of milk can stay safe without being refrigerated. (UHT milk contains fewer bacteria than conventionally pasteurized milk because it’s heated to a higher temperature. It’s also packaged in sterile, airtight containers. Therefore, unopened UHT milk can be stored without refrigeration for up to 90 days. Once opened, bacteria can get into the UHT milk, and it must be refrigerated.)
1- Design and Conduct Lab
- Ask students to form teams of three or four and encourage each team to develop a hypothesis on how temperature affects bacterial growth. Then ask them to design a lab experiment to test the hypothesis.
- Introduce the three materials teams must use for their lab: regular pasteurized milk, ultra-high-temperature (UHT) milk, and methylene blue, which indicates the oxygen level in a liquid.
- Ask, "How might you use methylene blue to help with your lab?" Students can research and discuss how oxygen levels in the milk might be related to the presence of bacteria. Tell them they can use any of the other materials on the lab table. Also, tell them that there’s a refrigerator and freezer they can use if you have one available.
- Let teams discuss their hypotheses and experimental designs for 10 to 15 minutes. Then, begin posing the following questions and discussion points to help students design well-thought-out labs:
- What do you know about the effect of temperature on bacteria? What are some ways you could test the effect of temperature on bacteria? (Heating is a way to kill bacteria; chilling and freezing are ways to slow the growth of bacteria.)
- Explain that one container of milk came from the refrigerated dairy case of the supermarket and the other from an unrefrigerated shelf. Let students examine each container.
- What’s an important difference between the two milk products? Is there any information on the labels that relates to our question about the effect of temperature on bacterial growth? (Students should discover that one is pasteurized and the other is UHT treated)
- What are the similarities and differences between pasteurized and UHT milk? (Both pasteurization and UHT treatment use heat to kill bacteria. UHT methods use higher temperatures than regular pasteurization. Also, UHT products are packaged in special airtight containers to prevent bacteria from getting into the product.)
- Could there be differences in the growth of bacteria between the two milks? What do you think the differences might be? (The regular pasteurized milk should show bacterial growth sooner than the UHT milk because the pasteurized milk has more bacteria in it.)
- Should you consider these differences when you design your labs? Why? (Yes, both milks should be tested in all conditions.)
- How can you tell if bacteria are growing in the test samples? (Add methylene blue to each sample. If bacteria are growing, the methylene blue will become colorless, and the milk will change from blue to white. This is not immediate but happens over time.)
- Have each group present its hypothesis and experimental design to the class. Encourage students to discuss the merits of each suggested test. (One effective experimental design is to test pasteurized milk and UHT milk at three temperatures — room temperature, chilled, and frozen.)
- After the group discussions, give the teams time to revise their hypotheses and experimental designs.
 Let teams conduct the labs according to their designs.
Let teams conduct the labs according to their designs.
- Note: The test tubes must be checked each day after the lab is started. Since the color change happens over time, you could miss important findings if you don’t check every day.
- Allow students to design their experiments, but the following steps can be used as a teacher reference:
- Assemble the test tube rack, and line up the tubes in the rack.
- Using the pipette or graduated cylinder, add 10 ml of the UHT milk into three test tubes. Repeat with the 2% pasteurized milk in the remaining three test tubes.
- Place a single drop of the methylene blue solution into each test tube. Be extremely careful with the solution—it will stain clothing and surfaces blue. Secure the caps and gently swirl the tubes to diffuse the dye evenly.
- Label the samples “UHT” or “2% Pasteurized.”
- To measure the effect of temperature on the rate of spoilage, keep one of each (UHT and 2% pasteurized) in the stand at room temperature, put one of each in the refrigerator, and one of each in the freezer.
- Observe the color of the milk over the next few days or weeks. The blue tint will begin to fade as the milk spoils. Record the color of each of the tubes according to the following scale:

- As the milk in the tubes becomes completely white students can begin to make a comparison and draw conclusions about how processing treatments deter bacterial growth.
2- Observe and Record Lab
- Pass out one copy of the Blue’s the Clue Data Table to each team or student.
- Beginning on day two, students should observe and record the visual changes they see in their milk samples.
- Option A: If observation time is short, you can speed the results by taking the refrigerated and frozen samples out of the freezer on day two and then observing them after sitting at room temperature for another day. Ask:
- "How did the data support or reject your hypothesis?
- "What might happen if the chilled and frozen samples were left out at room temperature for several hours or overnight?"
- "Should we test them to find out?" (Yes, let the chilled and frozen samples stand at room temperature until the following day. As they reach room temperature and remain in the Danger Zone for several hours, the bacteria will begin to grow. As this happens, the methylene blue will become colorless, and the milk will change from blue to white. Observe and record the results.)
- "What might happen if the UHT samples were left out at room temperature for another day?" (If you let the UHT samples sit out at room temperature for another day or more, the color will change to white. Observe and record the results.)
- Option B: If you have a longer period of time to observe results, continue daily observation of samples for up to 10 days or until the samples are all spoiled (white). This will allow students to see that milk (and other foods) can spoil in the refrigerator or after being frozen depending on the length of time they are there. Ask the following questions:
- "How did the data support or reject your hypothesis?"
- "How do time and temperature relate to food spoilage?" (The warmer the temperature, the quicker the spoilage. The cooler the temperature, the slower the spoilage.)
- Option A: If observation time is short, you can speed the results by taking the refrigerated and frozen samples out of the freezer on day two and then observing them after sitting at room temperature for another day. Ask:
3- Observe, Record, and Report Lab
- Observe and record findings on the final observation day. Ask students, "What happened to the frozen and chilled samples? What happened to the UHT samples?"

- Give students 5 to 10 minutes to complete their Blue's the Clue Data Tables.
- Have teams present their findings to the class. They should report both positive and negative results and discuss ways they would improve their experimental designs. Remind students to include the relationship of their findings to food safety.

Elaborate
-
Use the lesson FoodMASTER: Food Safety to explore the connection between microorganisms and food safety.
-
Further explore the role that microorganisms play in fermented foods using the lesson FoodMASTER: Milk and Cheese.
-
Take a 360 virtual field trip to a dairy farm.
-
Test UHT milk that has an expiration date that has passed and UHT milk that has an expiration date in the future. See if the “expired” milk changes more quickly than the fresher milk.
-
Compare the spoilage rate and bacterial growth in milk samples of varying fat content, such as 2%, whipping cream, and half-and-half.
-
Talk about sources of contamination, and compare contaminated samples with samples straight out of the milk jug. Possible sources of contamination can include a student taking a drink out of a glass and then testing the remaining milk, leaving the lid off of a test tube, putting a shoelace tip into a test tube of milk, etc. This can lend itself to a discussion about inoculation and contamination through which bacteria enters a system. In some occupations, people want certain bacteria to be present. Try making yogurt with your class as an example.
Evaluate
After conducting these activities, review and summarize the following key concepts:
- Food processing, such as the pasteurization of milk, makes food safer and more accessible because it can be transported longer distances if needed.
- Food scientists help develop and manage food processing practices that are safe and benefit consumers.
- Bacteria can be the cause of food contamination.
Use the following questions for evaluation:
- What do chilling, freezing, and heating do to bacteria? (Chilling and freezing slow down bacterial growth, but heating kills the bacteria.)
- Were bacteria in the milk killed at the different temperatures? Why or why not? How could you tell? (No. Only heat kills bacteria. Room temperature isn’t high enough to kill bacteria, and chilling and freezing do not kill bacteria but rather just slow their growth. When the chilled and frozen milk reached room temperature, bacteria began to grow again.)
- What is pasteurization? (Pasteurization is the process of destroying harmful bacteria that could cause disease by applying heat to a food; however, some spoilage bacteria may still be present.)
- What’s a basic difference between conventionally pasteurized and UHT milk? (Unopened UHT milk can be stored on a shelf without refrigeration for up to 90 days. Bacteria grow more quickly in regular pasteurized milk than in UHT milk because the latter uses higher temperatures, thus killing more bacteria. Also, UHT milk is sealed in sterile, airtight containers.)
- Explain the importance of knowing about the Danger Zone in food safety. (Awareness of the Danger Zone helps people understand the importance of heating and chilling food, thus decreasing the risk of foodborne illness.)
Acknowledgements
The Science and Our Food Supply Curriculum was brought to you by the Food and Drug Administration Center for Food Safety and Applied Nutrition and the National Science Teachers Association.
- FDA Education Team Leader Food Safety Initiative: Marjorie L. Davidson
- FDA Science and Our Food Supply Project Director: Louise H. Dickerson
- FDA/NSTA Associate Executive Director and Science and Our Food Supply Program Director: Christina Gorski
- FDA/NSTA Science and Our Food Supply Program Assistant: Jill Heywood
Option B of the lab activity was adapted by Debra Spielmaker and Utah Agriculture in the Classroom.
Recommended Companion Resources
- Beef Blasters
- Biotech Cheese Kit
- Blue's the Clue
- Dairy in the Mountain West: Our Family of Farmers
- Discover Dairy
- Food Chemistry Experiments
- Food Safety A to Z Reference Guide
- From Moo to You Video
- Gourmet Lab: The Scientific Principles Behind Your Favorite Foods
- Lose a Million Bacteria The Game
- Louis Pasteur and Pasteurization
- Make Mine Milk
- NMSU Field Trip: Milk
- The 12 Most Unwanted Bacteria
- The American Dairy Industry
- The Journey of Milk